Batteries – why and how they fail: Part 1

The first in a two-part series in which Andrew Pearce explores the life and times of batteries.
Some batteries die before their time, others, make it to a ripe old age. Either way, they have a finite life and can’t go on forever.
This two-part series explores the main types used in agriculture, explains why they fail and goes on to assess two bits of technology which, respectively, diagnose battery condition and attempt to recover seemingly dead items.
Most farm vehicle batteries use lead-acid technology.
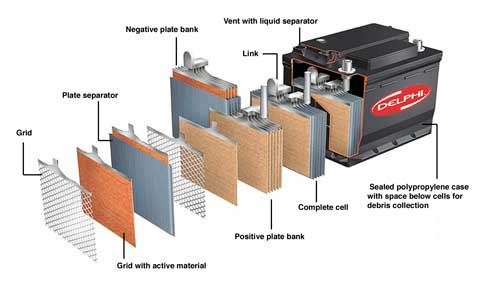
Whether a unit is destined for vehicle duty or for powering a live-stock fence, the basic construction is the same. Inside the casing are two sets of plates, one set positive and one negative, with separators between. The tops of the negative set link to the battery’s negative terminal or post, the positive ones join up to the positive terminal.
Lead skeleton
 |
|
Each plate is based on a lead skeleton, hardened by antimony or calcium. On the skeleton hangs a paste of lead oxide, which stiffens those bones and forms the battery’s active material. The separators between plates stop neighbours sort-circuiting, and have just the right degree of porosity to let charge-carrying ions move freely around. |
Normally at least six plates make up a single cell. When fully charged this produces just over two volts. So a 12V battery has six cells and shows around 12.7V between the terminals when fully charged, but not under load.
Conventional wet cells hold a mix of sulphuric acid and water. The battery stores electrical energy in chemical form on the plates. Charging a battery turns the active material on the positive plates to brown lead dioxide, and the negative plates to grey spongy lead.
When charge, electrical energy is stored in the plate materials and the concentration of sulphuric acid between them is high.
During discharge, electrical energy is released. Chemical change turns both plates’ active material to white-ish lead sulphate and swells them. The concentration and density of the sulphuric acid drops.
Lead-acid batteries split into two broad camps. It’s essential to sort out which is which to decide on application, charging procedure and fault-finding method.
Cranking batteries
 |
|
• Cranking batteries have many thin plates, a big internal surface area and are built to deliver high current for a short time, which is exactly what a starter motor wants. They can stand many such shallow discharges but they hate being almost flattened then recharge. The heat involved buckles the thin plates, and the substantial swelling/shrinkage that goes on in the active material tends to flake it off. Both these lower capacity, quickly and permanently.The message? Don’t use a cranking battery to power an electric fence (or anything else which demands a low, steady load) unless glory days are already over. |
Storage belt batteries
 |
|
• Deep discharge or storage belt batteries (aka deep cycle – or leisure types) have thicker plates. Used on boats, caravans, electric fence units and so on, they are built to supply power to a steady load and to be run almost flat, repeatedly and without damage. What don’t they like: Short-duration, heavy loads. Put one on engine-start duty and not only will cranking performance be lower, but you’ll also quickly kill it. |
These main types sub-divide again. You’ll need to sort one from another for charging and testing.
• Wet batteries are the conventional sort. Relatively cheap, they use lead-antimony plates; have liquid electrolyte and removable cell caps. Water leaves the electrolyte during charging so cells have to be topped up. Charging is conventional.
• Maintenance-free, sealed, gel or VRLA batteries use lead-calcium plate skeletons to reduce evaporation and gassing. Cell caps may be removable but not easily, as topping-up liquid electrolyte is not routine (1, 2).
Where the electrolyte is a gel there’s no spill risk and the battery can be used (but not charge) in different positions. Gases are vented through one-way valves.
Charge rate must be slow to limit internal temperature and gas production: conventional charges are usually wrong for the job. A ‘magic eye’ charge indicator (3) is another clue.
AGM batteries
 |
|
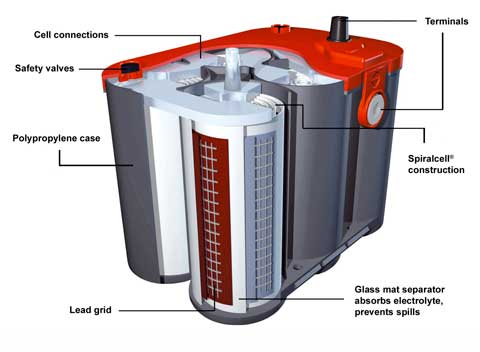
• Absorbed glass mat (AGM) batteries have fine fibre mat between the plates. This stops electrolyte swilling about, cuts shedding and stops evaporation so the battery can be effectively sealed. The result is potentially long life and high vibration tolerance. Plus plates can be rolled into a spiral. As a result internal resistance is particularly low and cranking performance excellent. Cells are still vented so charging voltage must be regulated. Conventional trickle or boost chargers will damage AGM batteries. |
 |
|
• Keep casings clean and dry. Current flows between battery posts through a layer of damp, corrosion or oil, increasing self-discharge and hence sulphation. Where battery construction allows, keep cells topped up. Exposed plate material permanently sulphates. |
Unused battery
 |
|
• Where a battery must be left in an unused machine for several weeks, disconnect the negative lead so small parasitic loads like a clock don’t slowly promote sulphation. Radios and such may need coding on reconnection, ECUs may lose stored settings. |
• Out of season, take the battery off an unused machine. Store cool and charge every couple of weeks.
• Invest in a charger designed to reverse sulphation and, once the batter is fully charged, hold it indefinitely in float or standby mode without over-charging.
 |
|
Failure is usually down to four things, acting alone or together. Old age in use, plate material slowly dissolves into the electrolyte. As this active material reduces, so battery capacity drops and cranking performance goes down. This may not be spotted in summer, but often shows up as an increasing reluctance to spin an engine as mornings get colder. All the while, dissolved material falls into the spaces below the cells. When it builds up enough to short-circuit the plates, the battery suddenly gives up the ghost and will crank hardly or at all. Expect around five years’ service from a good quality, well-looked after battery. |
Vibration
 |
|
Boost charging both cause plates to shed their material, the latter through rapid heating and shape change.Capacity drops, short-circuiting is likely. Overcharging The cells overheat, threatening, plate shape and structure; electrolyte boils off, capacity reduces. Sulphation Here’s the prime cause of early failure. During every normal discharge, material at the plates’ surface turns to inactive soft lead sulphate. It acts like an insulating blanket, steadily blocking access to the electrolyte and slowing chemical reaction down, and as a result the battery becomes discharged. Recharging usually splits up the soft sulphate, revitalises the electrolyte and the cycle starts again. Trouble begins when a battery is not fully recharged, is not kept topped-up with electrolyte or not used for several weeks – as with a combine or self –propelled forager that’s laid up through the winter. The battery naturally self-discharges so sulphation happens. If this is not reversed by thorough recharging, the normal soft sulphate turns into harder crystals which can’t readily be turned back into the soft form. The hard crystals lock on to the plates, reducing surface area and lowering battery capacity. First a sulphated battery slowly loses the ability to do its job, then won’t hold a charge and finally fails. In principle it’s easy to avoid permanent sulphation: use lead acid batteries regularly and charge them completely. |

